Ctrl + F is the shortcut in your browser or operating system that allows you to find words or questions quickly.
Ctrl + Tab to move to the next tab to the right and Ctrl + Shift + Tab to move to the next tab to the left.
On a phone or tablet, tap the menu icon in the upper-right corner of the window; Select "Find in Page" to search a question.
Share UsSharing is Caring
It's the biggest motivation to help us to make the site better by sharing this to your friends or classmates.
Physical Science
The fundamental principles and laws governing the natural world, topics such as physics, astronomy, and geology to understand the phenomena in our universe.
physics
chemistry
biology
astronomy
geology
thermodynamics
electromagnetism
kinetics
optics
quantum
genetics
atomic
molecules
energy
matter
Which of the following mirrors form virtual images --
- plane
- concave
- convex
- all the above
__________________ of solids can be separated based on property or magnetism. For example, chex mix can be separated, salad can be separated, a jar of random nails and screws can be separated
- Mixtures
- Solutions
On a graph showing distance versus time, a horizontal line represents an object that is
- Moving at a constant speed
- Increasing its speed
- Decreasing its speed
- Not moving at all
What is Newton's Third Law?

- F = ma
- For every action there is an equal and opposite reaction.
- Cheaters never prosper.
- Don't jaywalk.
Which of the following would occur as a result of unbalanced forces?
- The moon orbits Earth at a constant speed
- Twelve children leaning on a wall.
- A bus moves on the highway at 65 miles per hour
- Some kids fall playing tug-of-war
There is a faster rate of capturing neutrons before it undergoes radioactive decay.
- R-process
- S -process
What physical change of matter scatters
- Liquid
- solid
- gas
a certain molecule is composed of atoms that all pull on electrons with the same strength. Will the molecule be polar or non-polar?
- the molecule be nonpolar
It is a process that creates new atomic nucleus from pre existing nucleons, primarily electrons, neutrons and protons.
- NUCLEOSYNTHESIS
Blank is about the fusion of particles to form elements beryllium (Be-4) to iron (Fe-26).
- The Big Bang nucleosynthesis
- The Stellar nucleosynthesis
- The Supernova nucleosynthesis
This IMFA is an attraction between ion and polar molecules.
- Ion-Dipole Forces
- Dipole-Dipole Forces
- Hydrogen Bonding
- Dispersion Forces
There is less gravity on the ________
- Moon
It is referring to the phenomenon where the wavelength of the radiation from an object increases.
- Redshift
- Electroweak
- planck
- superforce
A driver slows down from 45 m/s to 20 m/s in 5 s. What is his acceleration?
- 5 m/s^2
- -5 m/s^2
- 25 m/s
- -25 m/s^2
11. Beneficial in removing hard water deposits, discoloration from aluminum, brass, bronze, copper and iron rust stains.
- Zonrox
- Acid
- Base
- Dishwashing Liquid
A car travels 22 km south, 12 km west, and 14 km north in half an hour.What is the final displacement of the car?
- 4 km
- 7 km
- 6 km
- 8 km
What is Newton's First Law?

- Equal and Opposite...
- F = ma
- An object in motion will stay in motion and obect at rest will stay at rest - unless acted upon by an outside force.
- Don't park in a fire lane.
Alchemy is came from arabic/ greek word _____means ______
- alkimiya means the art of transmuting
ionic bond
- a chemical bond formed between oppositely charged ions
- chemical bond in which two atoms share one or more pairs of valence electrons
- substances that undergo a chemical change
- difference between action between molecules of the same substance that they tend to stay together
There are three different states of matter: solid, ______, and gas

- liquid
He proposed that Earth is a disk floating on water.
- Thales of Miletus
Aside from an extremely high-temperature source, what is the other requirement for a supernova?
- electrons
- protons
- neutrons
- gravity
Which of the following is TRUE about nucleosynthesis?
- It is the process of creating new atomic nuclei from pre-existing nuclei.
- It comes from the word “nucleo”, meaning nucleus, and “synthesis”, meaning putting together.
- It is the creation of everything including all matter in the universe.
- All of the above.
His experiments on beryllium and alpha particles showed the existence of the third subatomic particle, the neutron, which is uncharged and located in the nucleus.
- Ernest Rutherford
- James Chadwick
- Neils Bohr
- John Dalton
describe two ways in an element can achieve a stable electron configuration
- by either transferring electrons or by sharing electrons
How to find the Charge of an Ion?
- no. of protons – no. of electrons
- Atomic mass – no. of electrons
- Atomic mass – no. of protons
- No. of protons + no. of neutrons
if a compound has 1 zinc atom and 2 chlorine atoms what is the compound's chemical formula?
- ZnCI2
When an object's distance from another object is changing.
- It is in motion
- It is speeding
- It has a high velocity
- It is acceleration
_________________occur when physical properties of a substance or object change.
- Physical changes
- Chemical changes
25% of the elements are blank while others are 2%.
- Hydrogen
- Uranium
- Helium
- Oxygen
covalent bond
- a chemical bond formed between oppositely charged ions
- a chemical bond in which two atoms share one or more pairs of valence electrons
- new substances formed as a result of a chemical change
- numbers that appear before a formula in a chemical equation
There is more gravity on the _________
- Earth
Image: https://quizizz.com/media/resource/gs/quizizz-media/quizzes/20af5b90-9d86-42fd-86f4-d10f44eb7eb3?w=200&h=200
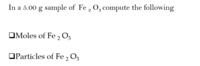
- MOLAR MASS Fe 2 O3 = 159.7 g/mol Fe2 O3
Determine if the proponent believed in geocentric or heliocentric model.ARISTARCHUS OF SAMOS
- geocentric model
- heliocentric model
Tycho Brahe is known for his geoheliocentric system.
- TRUE
- FALSE
What do you call the positively charged electron?
- atom
- positron
- neutron
- proton
When does condensation happen?
- Condensation happens when energy is removed gas turns to a liquid or liquid turns to a solid
- Condensation happens when something burns
The first three elements are blank.
- hydrogen
- helium
- lithium
- Iron
- Iron
A bicyclist travels 30 kilometers in two hours, her average speed is
- 30 km/h
- 60 km/h
- 15 km/h
- 2 km/h
cohesion
- the force of attraction between molecules of different substances that they tend to stick together
- the force of attraction between molecules of the same substance that they tend to stay together
- numbers that appear before a formula in a chemical equation
- a chemical bond in which two atoms share one or more pairs of valence electrons
Arrange the following in decreasing electronegativity: Al, Cl, P, Mg[Blank]
- Mg, Al, P, Cl
It is formed mainly from plants subjected to high temperature and pressure for millions of years
- Fossil Fuel
How do you measure Matter?
- ruler
- yardstic
- stick
- tube
- yardstick
what causes surface tension?
- adhesion
- cohesion
- ionic bonds
- covalent bonds
It is the type of bond happened between two atoms which are metals[Blank].
- Metallic
A theory that says the universe is developed from an infinitely, tiny, dense point that expanded rapidly.
- BIG BANG THEORY
The substance that the solute dissolves into is a ___________.

- solvent
- solute
- water glass
- solution
He performed experiments using his positive ray tube containing tubes with different gases at very low pressures with perforated tube and concluded that the positively charged particle in the atom is called proton.
- Neils Bohr
- Joseph John Thompson
- Eugene Goldstein
- Henry Moseley
The rate at which velocity changes is called
- Instantaneous speed
- Direction
- Acceleration
- Motion
Which of the following mirrors can form only virtual images ----
- convex
- plane
- both convex and plane
- concave
________ waves travel in straight waves.
- Light
- sound
- heat
A man traveling with his car 150 m to the east and then 70 m to the west, calculate the average velocity of the car if the travel takes 10 seconds.
- 6 m/s North
- 8 m/s East
- 10 m/s West
- 15 m/s South
Our universe started with a blank.
- Big Bang
Which of the following is the most accepted theory about the formation of the universe that explains why it continues to expand?
- Creation Theory
- Multiverse Theory
- Steady State Theory
- Big Bang Theory
___________ can change things physically by causing a change in state from solid, to liquid, to gas
- Heat
A car accelerates from rest to a speed of 36 km/h in 20 seconds. What is the acceleration of the car in m/s2?
- 0.1 m/s2
- 0.2 m/s2
- 0.5 m/s2
- 0.15 m/s2
The lower the viscosity, the slower the pouring.
- True
- False
Determine if the proponent believed in geocentric or heliocentric model.EUDOXUS OF CNIDUS
- geocentric model
- heliocentric model
Fluorine has the greatest electronegativity value while Cesium has the least value.
- True
- False
Determine if the proponent believed in geocentric or heliocentric model.NICOLAUS COPERNICUS
- geocentric model
- heliocentric model
They assigned certain symbols to match metals with the heavenly bodies such as the Sun and Moon.
- Chinese
- Arabs & Muslims
- Egyptians
- Mesopotamians
_________ energy is produced when objects rub against one another
- Heat
- Light
- Sound
They adapted techniques from the Mesopotamians and perfected the use of bronze, dye and glass that the Greeks later copied
- Egyptian
- Chinese
- Western Alchemy
- Mesopotamia
Describes as a violent explosion of the star that released a huge amount of nuclear energy.
- Big bang
- Radioactivity
- Nuclear Reaction
- Supernova
In this time, the universe's temperature cools down enough for electron to attached to nuclei. It is also the time where the second element formed.
- Hadron
- Lepton & Nuclear
- Atomic
- Galactic
What are the two other isotopes of hydrogen?
- diterium and tritium
- tritium and quadrium
- deuterium and quadrium
- deuterium and tritium
Where is potential energy the greatest in the roller coaster?
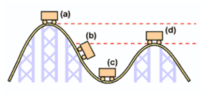
- A
- B
- C
- D
They especially focused on finding minerals, plants and substances that could prolong life.
- Egyptian
- Chinese
- Western Alchemy
- Mesopotamia
They enriched not only the practice but also the literature of chemistry. They also translated the practices and Aristotelian thinking of the Greeks and wrote extensively on how metals can be purified.
- Chinese
- Arabs & muslims
- Egyptians
- Mesopotamians
What are the 2 major stages happened after big bang explosion?
- Planck & Inflationary
- Electroweak & Nuclear
- Radiation & Matter
- Galactic & Stellar
The particles of matter making up a _______ are tightly packed together.
- Solid
- Liquid
- Gas
- Rock
_______ is measured from crest to crest or trough to trough of a wave.
- Wavelength
__________cannot easily be separated. Things DISSOLVE in a liquid quicker when heat is added, the substance is stirred, or when the area is spread out.
- Solutions
- Mixtures
________ are considered the building blocks of matter

- Nucleus
- Elements
- Particles
- Atoms
Determine if the proponent believed in geocentric or heliocentric model.CLAUDIUS PTOLEMAEUS
- geocentric model
- heliocentric model
______________ is every were
- Matter
What causes a balloon to remain inflated?
- The temperature of the air inside the balloon gradually decreases.
- Air particles collide with the walls of the balloon.
- The walls of the balloon gradually compress the gas inside.
- Air particles continue to enter through the balloon's walls.
Reactants
- substances that undergo a chemical change
- new substances formed as a result of chemical change
- a chemical bond formed between oppositely charged ions
This civilization learned to use acids such as nitric & sulfuric acids as well as nitro-hydrochloric acid
- Egyptian
- Mesopotamia
- Arab/Muslim
- Indian
The opposite of evaporation is __________.
- Condensation
- Convection
- Radiation
- Conduction
polar molecule
- a molecule that has slight positive and negative charges
He was considered the last & the greatest astronomer prior to the invention of the telescope
- Tycho- Brahe
What are the four ways you can change the state of matter?

- burning, cooking, steaming, and condensation
- melting, burning, vaporization, and freezing
- melting, freezing, vaporization, and condensation
- vaporization, melting, cooking, and freezing
___________spread out to fill the space they are in and move rapidly.
- Gases
- Liquid
- Solid
calcium carbonate is an ionic substance commonly called "chalk". If this molecule has one calcium atom (Ca), one carbon atom (C), and three oxygen atoms (O), what is the chemical formula?
- CaCO3
__________ is the ability of a fluid to exert an upward force on an object immersed in it.
- Viscosity
- Thermal expansion
- Buoyancy
- Vaporization
This resources can be used to reproduced biofuels like ethanol and biodiesels and biogas like biomethane. Ethanol and biodiesel can be mixed with or replace gasoline and diesel respectively.
- Biomass
What chemical family is being described when it contains 3 valence electrons, usually combined with oxygen and often used for glass wares and cleaning compound such as borax?
- Carbon Family
- Alkali Metals
- Nitrogen Family
- Boron Family
- *##**##*
Atoms are made up of the following parts: Nucleus, ______, Neutrons, and Electrons

- protons
A charge particle that orbits the nucleus.
- atom
- neutron
- electron
- proton
The ________________ tells the average mass of an atom of that element.

- Weight
- Atomic mass
- Atomic number
- Scale
Early Babylonians believed that Earth was spherical.
- TRUE
- FALSE
how many atoms of each element are in the compound AgNO3? (Ag=silver, N=nitrogen, O=oxygen)
- 1 silver, 1 nitrogen, 3 oxygen
A car travels 22 km south, 12 km west, and 14 km north in half an hour.What is the average velocity of the car?
- 1.5 m/s
- 2.5 m/s
- 3 m/s
- 3.5 m/s
The structure of an atom has important features. What are those? i. Some of these particles carry an electrical charge ii. Matter consists of indivisible particles iii. Atoms consists of smaller particles. iv. Atoms of the same element have identical set of properties.
- I, ii and iii
- Ii and iv
- I and iii
- ii only
Which of the following best describes particles in a solid?
- Molecules slide past each other; sample takes shape of container
- No attractive forces between particles
- Fills whatever container it is in
- Particles tightly packed together
It refers to the actual measure of the difference between initial positions up to the final position.[Blank]
- Displacement
It is where the star, the Sun and other heavenly bodies are embedded
- Celestial Sphere
Draw a force Diagram of Forces 2N to the left, 3 N to the right, 5 N up and 4 N down
- [No Answer]
Heavier elements than uranium are called blank.
- transuranium elements
- uranium elements
- supernova elements
He asserted that Earth spins on its axis every day & revolves around the Sun.
- Copernicus
(how something feels),
- color, texture
- Volume
- Mass
Matter can be described by it’s
- physical properties
- Chemical physical properties
- Gas physical properties:
Physical science is the study of _______ and _________.
- space and volume
- matter and energy
- temperature and time
- matter and space
Galileo confirmed that there were mountains on the moon using his telescope with 8x magnification.
- TRUE
- FALSE
Is this a good Quiz ?
- [No Answer]
6. It is a physical substance that is used to make things.
- Molecules
- Atoms
- Material
- Matter
A particle which has positive charge
- Electrons
- Neutrons
- Protons
- ions
Refrigerators use the process of __________ to remove heat from the interiors.
- Combustion
- Compression
- Sublimation
- Evaporation
Changing state is an object
- True
- False
(how much space something takes up),
- volume
- Mass
- Solid
What physical change of matter scatters a little
- Liquid
- solid
- gas
1. The attractive forces present between molecules.
- Intermolecular Forces
- Normal Force
- Air Force
- Atomic Force
Matter s is_____________ that takes up space and has mass! ________________ is matter! The smallest part of all matter is the atom!
- Anything & everything
He was one of the greatest Greek philosophers. He was the founder of Academy. He was the writer of the Republic and proponent of Platonic method of questioning
- Anaxagoras
- Democritus
- Plato
- Empedocles
Is the change in velocity in particular time interval?
- Acceleration
This IMFA is observed when hydrogen attracted to fluorine, oxygen and nitrogen, in which bond produces a very strong dipole force.
- Ion-Dipole Forces
- Dipole-Dipole Forces
- Hydrogen Bonding
- Dispersion Forces
Concave mirror form --------------------- and ---------------- images.
- real and virtual
Heavier elements than uranium are called transuranium elements, which are also called blank Elements.
- Synthetic
- Asynthetic
- Cynthetic
He was a Greek philosopher of nature remembered for his cosmology and for his discovery of the true cause of eclipses
- Anaxagoras
- Democritus
- Plato
- Empedocles
12. What element is generally used in common bleaches in household products?
- Chlorine
- Benzene
- Sodium
- Iron
A mountain climbing expedition establishes a base camp and two intermediate camps, A and B. Camp A is 11,200 m east of and 3,200 m above base camp. Camp B is 8400 m east of and 1700 m higher than Camp A. Determine the displacement between base camp and Camp B.
- 15 642 m
- 18 240 m
- 20 200 m
- 24 520 m
He was a student of Socrates & teacher of Aristotle. He wrote The Republic. He also expanded Empedocles' theory
- Anaxagoras
- Plato
- Democritus
- Aristotle
He adopted the Pythagorean view of the motion of the heavenly bodies as combination of circular motion about Earth.
- Pythagoras
Is this quiz hard?
- [No Answer]
He believed that there are smallest part of matter and can be further divided infinitely into smaller pieces.
- Aristotle
- Democritus
- Empedocles
- Galileo Galilei
Speed equals distance divided by
- Time
- Velocity
- Size
- Motion
John walks from the point A to B to C. What does the distance he travels?

- 9 m
- 7 m
- 13 m
- 5m
If a plane travels 48,000 m in 80 s, how fast is it going?
- 3840000 m/s
- 600 m/s
- 80 m/s
- 60 m/s
This is the forces that all molecules have on each other but this IMFA are evident in non-polar molecules.
- Ion-Dipole Forces
- Dipole-Dipole Forces
- Hydrogen Bonding
- Dispersion Forces
When the temperature of a substance is lowered, its particles __________ .
- Vibrate more slowly
- Escape the attractive forces of the other particles
- Stop vibrating completely
- Vibrate more quickly
why do ionic compounds include at least one metal in one nonmetal?
- because ionic bonds are formed between a cation, which is usually a metal,and an anion, which is usually a nonmetal
What do you call the relative ability of a bonded atom to attract shared electron pairs?
- Ionization energy
- Electronegativity
(measure of amount of space – similar to weight),
- mass
- Volume
- Color
John walks from the point A to B to C.What is the displacement?

- 5 m
How long will it take a Pony Express rider to travel 24,000 m if he is traveling at a speed of 12 m/s?
- 48,000 s
- 4,800 m
- 200 s
- 2,000 s
Images that can not be caught on screen are known as --------------------------
- virtual images
based on their chemical formulas, which of these compounds is not likely to be an ionic compound: SO2, KBr, or FeCI3? How can you tell?
- SO2 is not an ionic compound because they are two nonmetals which makes it a covalent bond
one of the most common household cleaners is ammonia, which has a chemical formula of NH3. how many atoms are in the molecule of ammonia?
- four
- three
- two
- five
This type of IMFA only acts between polar molecules.
- Ion-Dipole Forces
- Dipole-Dipole Forces
- Hydrogen Bonding
- Dispersion Forces
He was the first to use the telescope to observe astronomical phenomena in 1609.
- Galileo Galilei
products
- the force of attraction between molecules of different substances to that they tend to stick together
- new substances formed as a result of a chemical change
- the force of attraction between molecules of the same substance that they tend to stay together
- a molecule that has slight positive and negative charges due to an imbalance in the way electrons are shared
R = --------
- 2f
- f
- f/2
- none of above
John drove South 120 km at 60 km/h and then East 150 km at 50 km/h. Determine the average speed for the whole journey?
- 54 km/h
- 65 km/h
- 70 km/h
- 80 km/h
There are two valence electrons shared in a single bond formed.
- True
- False
According the Newton's Second Law, what factors affect force?
- speed and power
- shape and material
- mass and acceleration
- mass and weight
Inertia says that the heavier an object is, the _________ it is to move.

- easier
- harder
- smarter
- more important
________waves are created from vibrations
- Sound
- heat
- light
A force is a _________________ or __________________.
- Tug, spring
- Push, shove
- Push, pull
- Movement, stillness
9. A substance that increases the rate of a chemical reaction without itself being consumed by the reaction.
- Solution
- Solvent
- Catalyst
- Solute
Arrange the following in increasing electronegativity: Li, F, Na,C[Blank]?
- F, C, Li, Na
Weight equals
- Mass x time
- time x weight
- Gravity x friction
- Mass x gravity
To determine the acceleration rate of an object, you must calculate the change in speed during each unit of
- Velocity
- Time
- Motion
- Deceleration
Determine if the proponent believed in geocentric or heliocentric model.ARISTOTLE
- geocentric model
- heliocentric model
This is how three Helium-4 is converted into Carbon in a red giant star.
- Proton-proton chain
- CNO cycle
- Alpha ladder process
- Tri- alpha process
_________ have a definite shape (like an ice cube).
- Solids
- Liquids
- Gases
Which of the following intermolecular forces of attraction is arranged from strongest to weakest?
- H- bonding- dipole-dipole- London forces
- London forces- dipole-dipole- H-bonding
- Dipole-dipole- London forces- H-bonding
- H-bonding- London Forces- dipole-dipole
How many valence electrons are there in Iodine?
- 7
Where is the greatest kinetic energy?
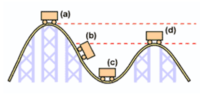
- A
- B
- C
- D
The 5th element that refers to heavinly bodies according to Aristotle.
- AETHER OR ETHER
Which process is responsible for the formation of light elements such as Hydrogen and Helium?
- Big Bang nucleosynthesis
- Stellar nucleosynthesis
- Supernova nucleosynthesis
- None of the above
Who was the inventor of the telescope?
- Hans Lippershey
__________ is the thermal energy that flows from a material with a higher temperature to one with a lower temperature.
- Kinetic energy
- Work
- Heat
- Potential energy
there are more than _______ different atoms
- 100
suppose you are able to count the molecules in a substance. Which would have more molecules, one liter of liquid water or 1 liter of ice? Why?
- water is denser than ice, so liter of water weighs more than a liter of ice, once the liter of water freezes, it's volume is greater than 1 liter. molecules are farther apart in ice molecules, and denser
There is a huge number of free neutrons available, so the time to capture a neutron is much shorter than the decay time.
- R-process
- S -process
In 1910, Slipher & Wirtz measured the wavelength of light from spiral nebulae it Explains that when wavelength of light increases due to the distance it travels increases. What is it?
- Redshift
What is the relationship of environment to the community?
- [No Answer]
What physical change of matter keeps form
- Liquid
- solid
- gas
73% blank makes up the majority of the elements found in the universe.
- Hydrogen
- Uranium
- Helium
- Oxygen
balance the equation CH4 + O2--------- CO2 + H2O
- CH4 + 2O2 -------- CO2 + 2 H2O
In an acceleration graph showing speed versus time, a straight line shows the acceleration is
- Decreasing
- Increasing
- Changing
- Constant
It is referring to the change in wave frequency during the relative motion between a wave source and its observer
- Redshift
- Doppler
- wavelength
- Isotopes
According to Pascal, pressure applied to a fluid __________ .
- Increases as it moves through the fluid
- Fluctuates as it is transmitted throughout the fluid
- Is transmitted unchanged throughout the fluid
- Decreases as it moves through the fluid
If I drive from Pantukan to Mati City at 40 miles per hour and then from Mati City to Pantukan at 60 miles per hour, what is my average speed for the whole journey?[Blank]
- 48 mph
Look at the picture given below. An object moves from point A through B, C, D, E and stops at point F.Find final displacement[Blank]. Find distance taken from point A to D[Blank].

- 8 m
- 20 m
A ____________ can be used to show how two elements share electrons in covalent bonding
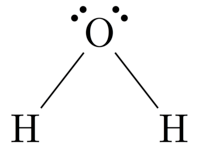
- Chemical reactions
- Proton dot diagram
- Electron dot diagram
- Chemical formula
He arranged the elements and greatly help us today with the Periodic Table.
- John Dalton
- Dmitry Mendeleev
- Joseph Proust
- Robert Millikan
This civilization perfected the use of iron & steel and were known for manufacturing dyes, glass, cements, solutions for textiles and soap
- Egyptian
- Mesopotamia
- Arab/Muslim
- Indian
Having a dam/ power plant nearby, is it really helpful? why?
- [No Answer]
The universe is composed of a total of blank elements from hydrogen to uranium.
- 92
He designed the first periodic table and arranged into an interval of eight elements[Blank].
- Dmitri Mendeleev
Francis traveled 360 km at an average speed of 80km/h. Nikka took 1.5 hours more to complete the same amount of distance. What was Nikka's average speed for the whole journey?
- 60km/h
Which of following mirror can not form real images -------------
- plane
- convex
- both plane and convex
- concave
Thermal energy is the __________ of the particles in a material.
- Potential energy
- Total energy
- Temperature
- Average kinetic energy
What is Newton's Second Law?

- Force = Mass x Acceleration ( F = ma )
- Equal and Opposite
- The Law Of Inertia
- Everybody loves the beach in Summer.
In this era, all of the universe’s ingredients were present, however universe is too hot and dense to create subatomic particles to form.
- Quark
- Inflationary
- Grand Unified
- Planck
Changing direction is an example of a kind of
- Acceleration
- Speed
- Velocity
- Constant rate
It refers to an energy released or required when electrons are added to an atom in the gaseous phase[Blank].
- Electron Affinity
In this Epoch, the first element were formed by fusion of proton & neutron
- Lepton & Nuclear
- Quark & Electroweak
- Galactic & Stellar
- Inflationary & Planck
The point on the principal axis where all the normals meet is known as ----
- center of curvature
- principal axis
- pole
- normal
According to Boyle's law, at a constant temperature, if the volume of a container of gas is __________, then pressure of the gas will __________ .
- Increased; increase
- Increased; decrease
- Decreased; decrease
- Decreased; increase
Can a solid melt at 0 degrees celsius
- yes
- no
Blank is the most abundant element.
- Hydrogen
- Uranium
- Helium
- Oxygen
The substance that dissolves in a solution is called a _____________.

- solvent
- solution
- solute
- mixture
The ________ is a star cluster that is visible to the unaided eye.
- PLEIADES
The following mirror is used as shaving mirror -----
- concave
- convex
- plane
- none of the above
The building blocks of all matter.
- atom
- neutron
- electron
- proton
__________ is the ability of a substance to dissolve in another substance.

- mixture
- solubility
- chemical reaction
- chemical formula
Images that can be caught on screen are known as -------------------
- real images
________________ occur when the chemical make up of a substance changes. For example, a piece of wood is burned. The chemical make up changes from wood to ashes.
- Chemical changes
- Physical Change
adhesion
- the force of attraction between molecules of different substances that they tend to stick together
Average speed is
- Equivalent to velocity
- The rate at which an object is moving at a given instant
- The rate at which a slope changes
- The total distance traveled divided by the total time
Air Resistance is ____________ acting on something moving through the air.
- Gravity
- Friction
- Wind
- Force
Who developed the Law of Triads?
- Alexander Beguyer de Chancourtois
- Dmitri Mendelev
- Johann Wolfgang Dobereiner
- John Newlands
It is an energy released or required when electrons are added to an atom in the gaseous phase.
- Electronegativity
- Electron affinity
- Atomic size
- ionization energy
Elements are believed to have existed blank.
- billions of years ago
- hundred of years ago
- ten of years ago
- million of years ago
Compute for Alpha decay

- W
A __________ is a mixture that looks like a single substance and has the same properties throughout.
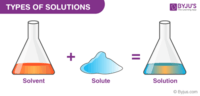
- jello
- solvent
- solute
- solution
He discovered & explain the true cause of eclipses
- Empedocles
- Plato
- Democritus
- Anaxagoras
coefficients
- numbers that appear before a formula
What is the ultimate fate of our Sun?
- to become a planetary nebula
- to become a white dwarf
- to become a red giant star
- to become a massive star
Produced elements up to Fe.
- The Big Bang nucleosynthesis
- The Stellar nucleosynthesis
- The Supernova nucleosynthesis
There are several forms of energy: light, heat (thermal), mechanical, sound, electrical, and chemical
- light and chemical
- heat (thermal),
- mechanical
- sound,
- electrical
Which of the following analogy is TRUE?
- Big Bang nucleosynthesis: heavier elements starting from iron.
- Stellar nucleosynthesis: lighter elements starting from iron.
- Supernova: heavier elements starting from iron.
- Big Bang nucleosynthesis: lighter elements up to iron.
Gravity is the greatest where?
- The Earth
- The Moon
- Jupiter
- The Sun
Which of the following mirror is used as rear-view mirror ---
- concave
- convex
- plane
- none of the above
A Greek philosopher who says that matter is from 4 elements namely fire, air, water and earth.
- Aristotle
- Empedocles
- Democritus
- Galileo Galilei
Matter can make a physical change
- True
- False
The supporting force exerted by a fluid on an object immersed in it is called __________.
- Lift
- Denisty
- Buoyant force
- Viscosity
Why do average stars have a longer life span than massive stars?
- They have more fuel to burn.
- They have less fuel to burn.
- They burn their fuel at a faster rate.
- They burn their fuel at a slower rate.
Two or more elements that combine are called a ___________.
- Compound
- Chemical bond
- Ionic bond
- Covalent bond
According to the kinetic theory, all matter is composed of __________.
- Solid material
- Waves
- Plasma
- Particles
what is responsible for water being a liquid at room temperature as well as for water's properties of cohesion and adhesion?
- water's polarity and hydrogen bonding
This is the force on an object due to gravity.
- force applied
- force normal
- force weight
- force friction
Venus is considered the second brightest in the sky.
- TRUE
- FALSE
The moving particles in an object have __________ energy.
- Decreasing
- Increasing
- Kinetic
- Potential
He arranged the elements in increasing atomic mass.
- Dmitri Mendelev
- John Newlands
- Glen Seaborg
- Henry Moseley
________take the shape of their container and move a little
- Liquids
- Gases
- Solid
What chemical families is being describe if it has four valence electrons?
- Nitrogen Family
- Alkali Family
- Boron Family
- Carbon Family
do you want these quizizz to be harder?
- yes
- no
Which of the following intermolecular forces of attraction is considered the weakest?
- H -bonding
- ion-dipole
- dispersion forces
- dipole-dipole
This process happens in massive stars which convert Hydrogen into Helium.
- Proton-proton chain
- CNO cycle
- Alpha ladder process
- Tri- alpha process
Arrange the following in decreasing atomic size: S, Ca, F, Rb, Si[Blank]?
- Rb, Ca, Si, S, F
A place or oject used for comparison to determine if something is in motion is called
- A position
- A reference point
- A constant
- Velocity
Stellar nucleosynthesis stops at the element of iron because there are blank in their nuclei.
- not enough energy
- not enough protons
- not enough electrons
- not enough neutrons
He was the one who he stated that everything is made up of four eternal and unchanging kinds of matter
- Anaxagoras
- Democritus
- Plato
- Empedocles
He suggested that Earth was moving around the Sun, but Aristotle and most of the ancient Greek scholars rejected this idea.
- Aristarchus of Samos
What is the building block of all elements (except for hydrogen) ?
- Helium
- Lithium
- Carbon
- Iron
It is a member of a family of an element that all have the same number of protons but different numbers of neutrons
- Redshift
- Doppler
- wavelength
- Isotopes
Compute for alpha decay

- Pb
Orion is represented by ______ , god of death, rebirth, and afterlife.
- Osiris
what happens to the charge on atoms when they form a polar covalent bond?
- making some partial electric charges, the atom with the greater attraction for electrons has a partial negative charge, the other atom has a partial positive charge which has a relatively less attraction for electrons
- it makes them all electric charges
- making all of them them have an electric charge the atom with the lesser attraction for electrons has a partial positive charge, the other atom has a partial negative charge and which has a relatively greater attraction for electrons
- it forms a polar ionic bond
Distance (height) and mass determine the amount of _________ energy in an object.
- Kinetic
- Potential
- Solar
- Thermal
It is the type of bond happened between a metal and nonmetal atoms[Blank].
- Covalent
You measure Mass with ___________
- Balance
You way more on the Moon than the Earth
- True
- False
13 - 15. Give 3 examples or personal care products used to enhance the appearance of human body
- [No Answer]
You way more on Earth the than the Moon
- True
- False
When you know both the speed and direction of an object's motion, you know the
- Average speed of the object
- Acceleration of the object
- Distance the object has traveled
- Velocity of the object
Liquids can form spherical elastic film to minimize surface area. What intermolecular forces are responsible for the formation of this film in water?
- H -bonding
- ion-dipole
- dispersion forces
- dipole-dipole
The higher viscosity, the faster the pouring.
- True
- False
What happens when a force becomes unbalanced?
- Nothing
- Explosion
- Silence
- Movement
2-5. Enumerate the four (4) macromolecules
- Carbohydrates Lipids Protein Nucleic Acids
8. A theory whose premise is : molecules have to collide to react.
- Theory of Evolution
- Collision Theory
- Constitutional Theory
- Lewis Theory
Through their unexpected discovery, they supported the NASA'S project "Echo". They concluded that the noise CMB radiation the remains energy created after the expansion
- Socrates & Democritus
- Aristotle & Plato
- Wilson & Penzias
- Anaxagoras & Empedocles
This ancient civilization can be found near 2 rivers. They made use of other materials such as dyes, glass, paints & perfumes
- Egyptian
- Mesopotamia
- Arabs/ Muslims
- Indians
It is the force that can attract or repel particles.
- Gravity
- Electromagnetic force
- Strong force
- Weak force
Produced the light elements – H, He and few Li.
- The Big Bang nucleosynthesis
- The Stellar nucleosynthesis
- The Supernova nucleosynthesis
A Russian chemist organized all the known elements into what is known as the __________________________.

- Periodic table of dogs
- Periodic table of mass
- Periodic table of elements
- Periodic table of families
A ________________ is a way to describe a chemical reaction using chemical formula.
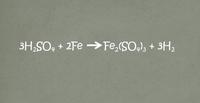
- Reactant
- Chemical properties
- Chemical equation
- Mixture
What charge of an atom can be observed in a polar molecule?
- Two positively charged atoms
- Two negatively charged atoms
- Neutral atom
- One positively charged and one negatively charged atom
10. Give one way on how do humans harness energy
- Fossil Fuels
He used the Redshift of light from the galaxies to calculate the velocities and distances of these galaxies from the Earth.
- Edwin Hubble
What type of bond is being formed between Li and O[Blank]?
- Ionic
Which way a dipole arrow is pointed to?
- A more positively charged atom.
- An atom that is least electronegative.
- An atom that is more electronegative.
- All of the above.
There is a small number of free neutrons available, so the time to capture a neutron is much longer than the decay time.
- R-process
- S -process
If the particles in an object begin to move more quickly, their average kinetic energy __________ and the object's temperature __________.
- Decreases, rises
- Increases, falls
- Increases, rises
- Decreases, falls
How elements heavier than iron are formed?
- Solar nucleosynthesis
- Big bang nucleosynthesis
- Stellar nucleosynthesis
- Supernova nucleosynthesis
The center of the deferent is called _______.
- eccentric
They perfected the use of bronze, dye and glass that the Greeks later copied
- Chinese
- Arabs & Muslims
- Egyptians
- Mesopotamian
Which of the following situation describes capillarity in liquid?
- Marble easily plunges into the water.
- Liquid mercury rises easily in a narrow tube.
- Acetone easily flows in a sliding direction.
- Water evaporates after a day-long exposure to sunlight.
Identify the predominant intermolecular forces present between Carbon dioxide (CO2) and methane (CH4).
- H -bonding
- ion-dipole
- London dispersion
- dipole-dipole
It is formed when electrons are removed or added to a neutral atom.
- Isotopes
- Cation
- Nuclide
- Ions
Which of the following is NOT part of Galileo's discoveries using his telescope?
- lunar craters
- sunspot
- free fall
- moons of Jupiter
Blank elements are least abundant.
- Heavier
- Lighter
7. It is when a reaction involving the rearrangement of atoms occurs
- Products
- Substances
- Chemistry
- Chemical Change
Which of these is an example of deceleration?
- A bird taking off for flight
- A roller coaster moving down a steep hill
- A car approaching a red light
- An airplane following a straight path
During this time universe is rapidly expanded from the size of an atom to a size of a fruit. Also during this time, the universe is super-hot to the point that it created with electron and other particles.
- Quark
- Inflationary
- Grand Unified
- Planck
According to Charles's law, at a constant pressure, if the temperature of a gas __________, the volume __________ .
- Increases; increases
- Decreases; decreases
- Increases; decreases
- Decreases; increases
If you put a solid in at 32 degrees fare in and height, will it freeze?
- yes
- no
Water is classified as a polar molecule.
- True
- False
To keep up this site, we need your assistance. A little gift will help us alot.
Donate- The more you give the more you receive.
Related SubjectThe Solar System
Physics
Physics For Engineers
Environmental Science
Earth Science
Chemistry for Engineers
Calculus-Based Physics
Chemistry
Inorganic and Organic Chemistry
General Chemistry Organic
Healthcare Studies
Tadpoles: From Water to Land
Cell Theory
Life Science
Microbiology and Parasitology
Anatomy and Physiology
Planet Parade in the Night Sky
Astronomical Events 2025
Space Exploration 2025
Leonid Meteor Shower Peaks in November
Beaver Moon in Science
The Science of SpaceX Satellites
Exploring the International Space Station
The Taurids Meteor Shower: What You Need to Know for 2024
The Ancient History of Sunlight
Avalanche Studies
Mt. Kanlaon: A Study of the Volcano
Kinesiology
Quantum Computers
Triple-Negative Breast Cancer
Health Sciences
Biology
Biological Science
Rapidly Feeding Black Hole Discovery
Germany Energy Policy
Adipose Tissue
Show All Subject
Affiliate Links
Shopee Cashback Voucher
Temu $0 Shipping Fee
Amazon 75% Off Discounts